Publications
2026
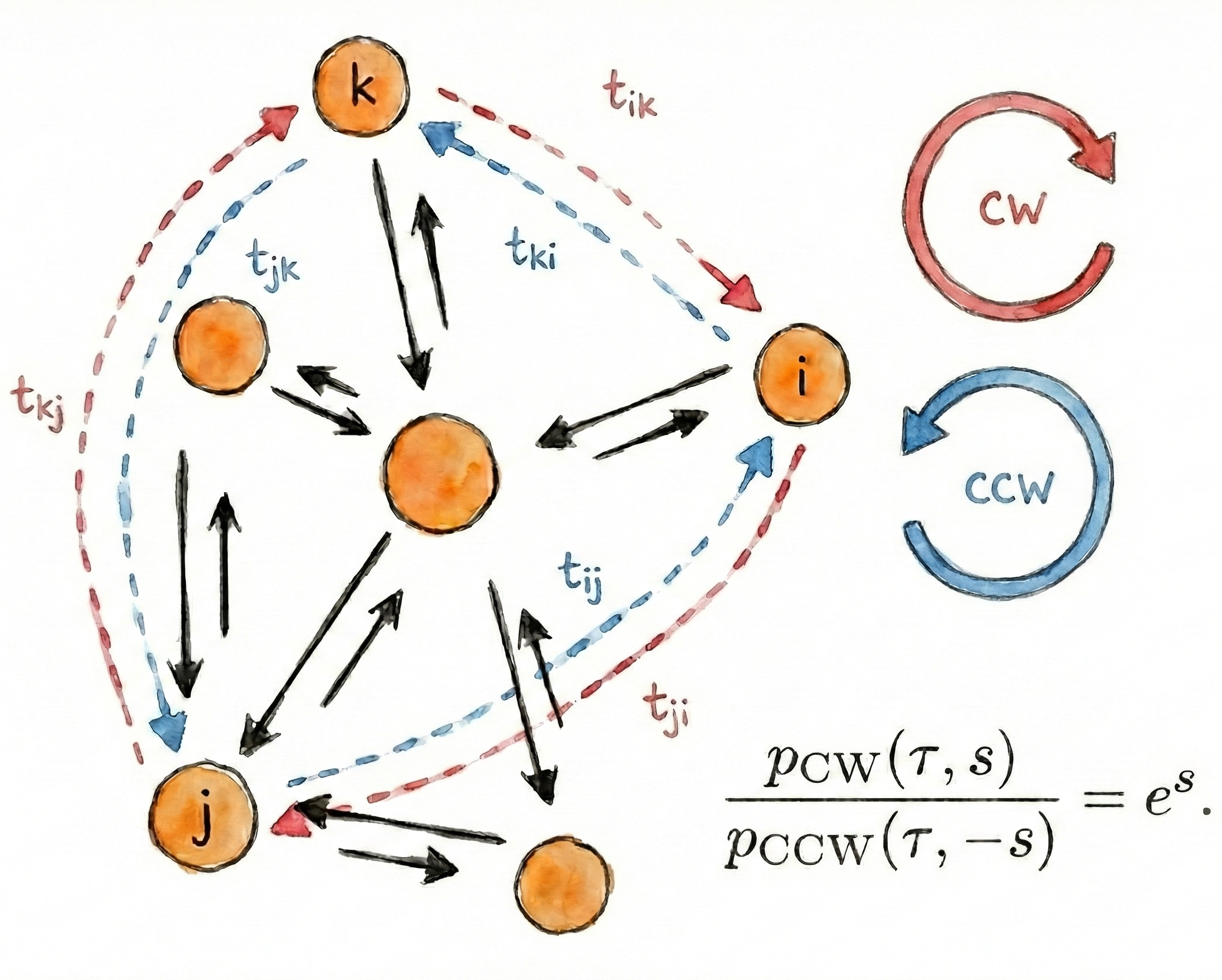
Non-equilibrium Symmetry of Cyclic First-Passage Times
D.M. Busiello, S. Liang & S. Pigolotti
arXiv:2601.18136 (2026)
$\color{red}\blacksquare$ A fluctuation theorem for cyclic first-passage times, providing a new indicator for detecting nonequilibrium in complex systems.
2025

Stochastic Thermodynamics for Biological Functions
Y. Cao & S. Liang
Quantitative Biology 13(1), e75. (2025)
$\color{red}\blacksquare$ A review on recent advances of stochastic thermodynamics and application to biological functions.
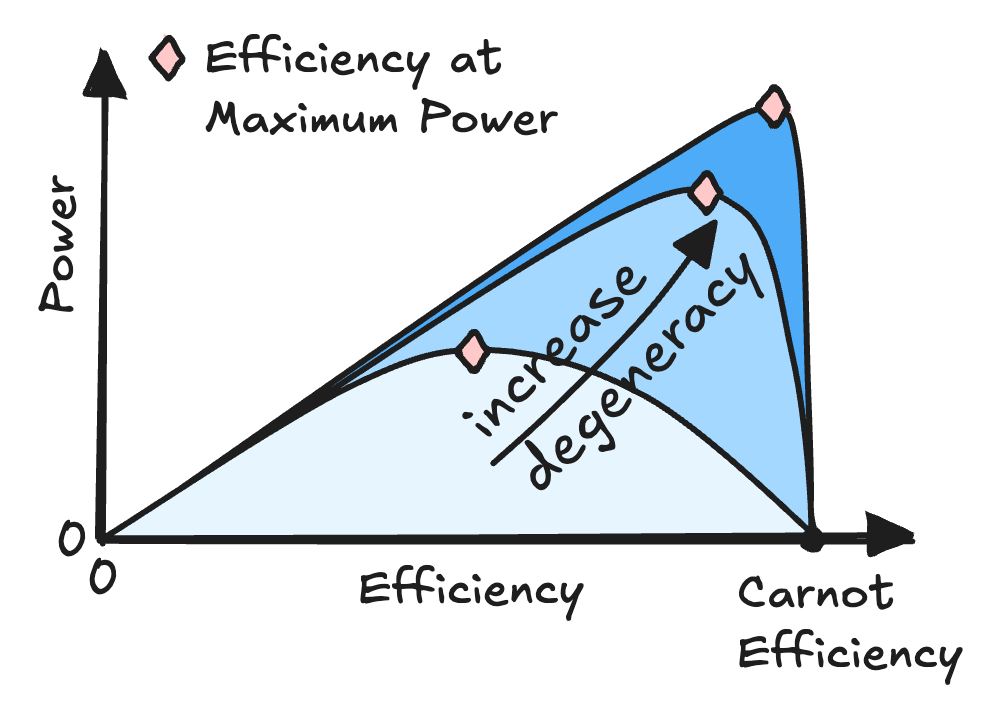
Minimal Model for Carnot Efficiency at Maximum Power
S. Liang, Y.-H. Ma, D. M. Busiello & P. De Los Rios
Phys. Rev. Lett. 134, 027101 (2025) | arXiv 2312.02323 (2023)
Media Coverge: Phys.org | Interesting Engineering | South China Morning Post
$\color{red}\blacksquare$ A minimal model reveal the attainability of Carnot efficiency at maximum power.
2024
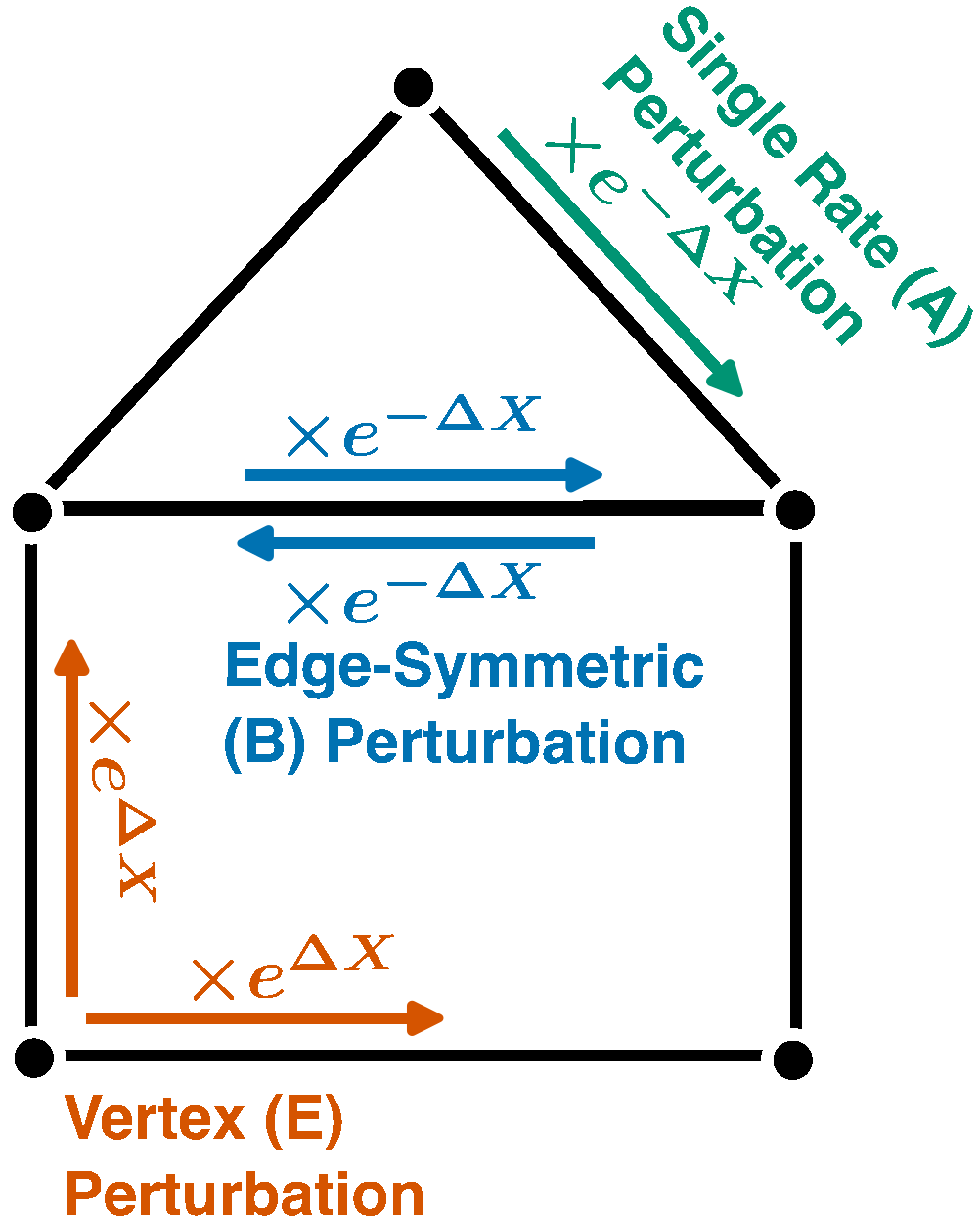
Nonequilibrium Response Theory: From Precision Limits to Strong Perturbation
R. Bao & S. Liang
arXiv: 2412.19602 (2024)
$\color{red}\blacksquare$ Equalities and bounds for (nonequilirbium) nonlinear resposne of Markov chains under strong perturbation.
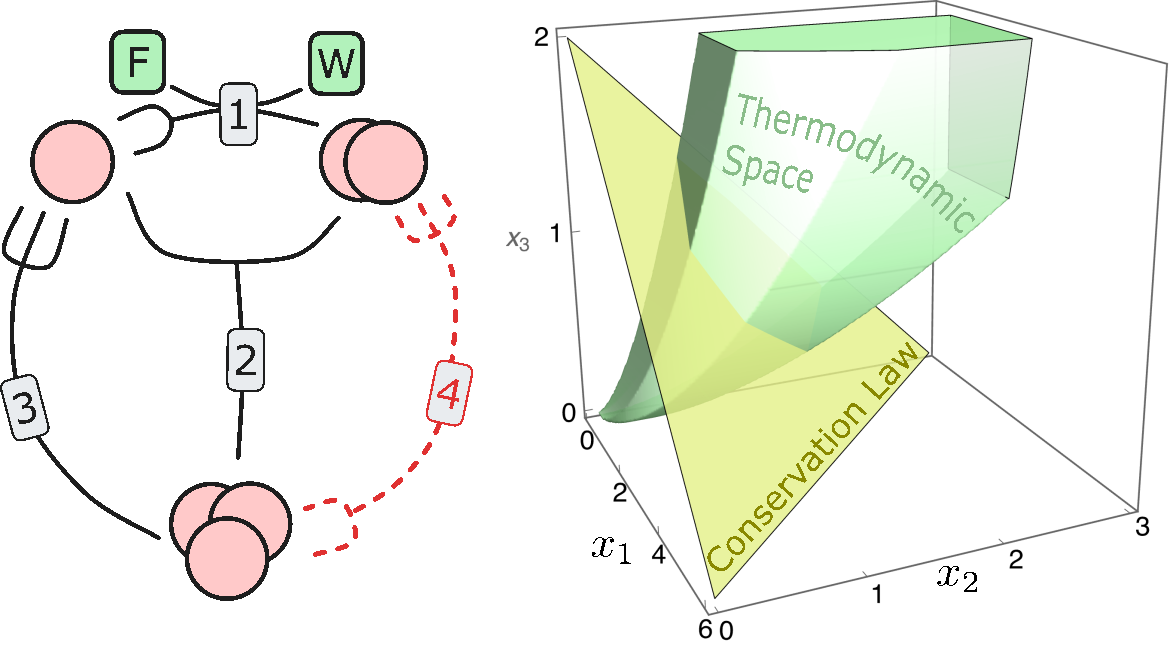
Thermodynamic Space of Chemical Reaction Networks
S. Liang, P. De Los Rios & D. M. Busiello
arXiv: 2308.14497 (2024)
Video
$\color{red}\blacksquare$ Thermodynamically accessible phase space for (non-equilibrium) chemical reaction networks at stationarity.
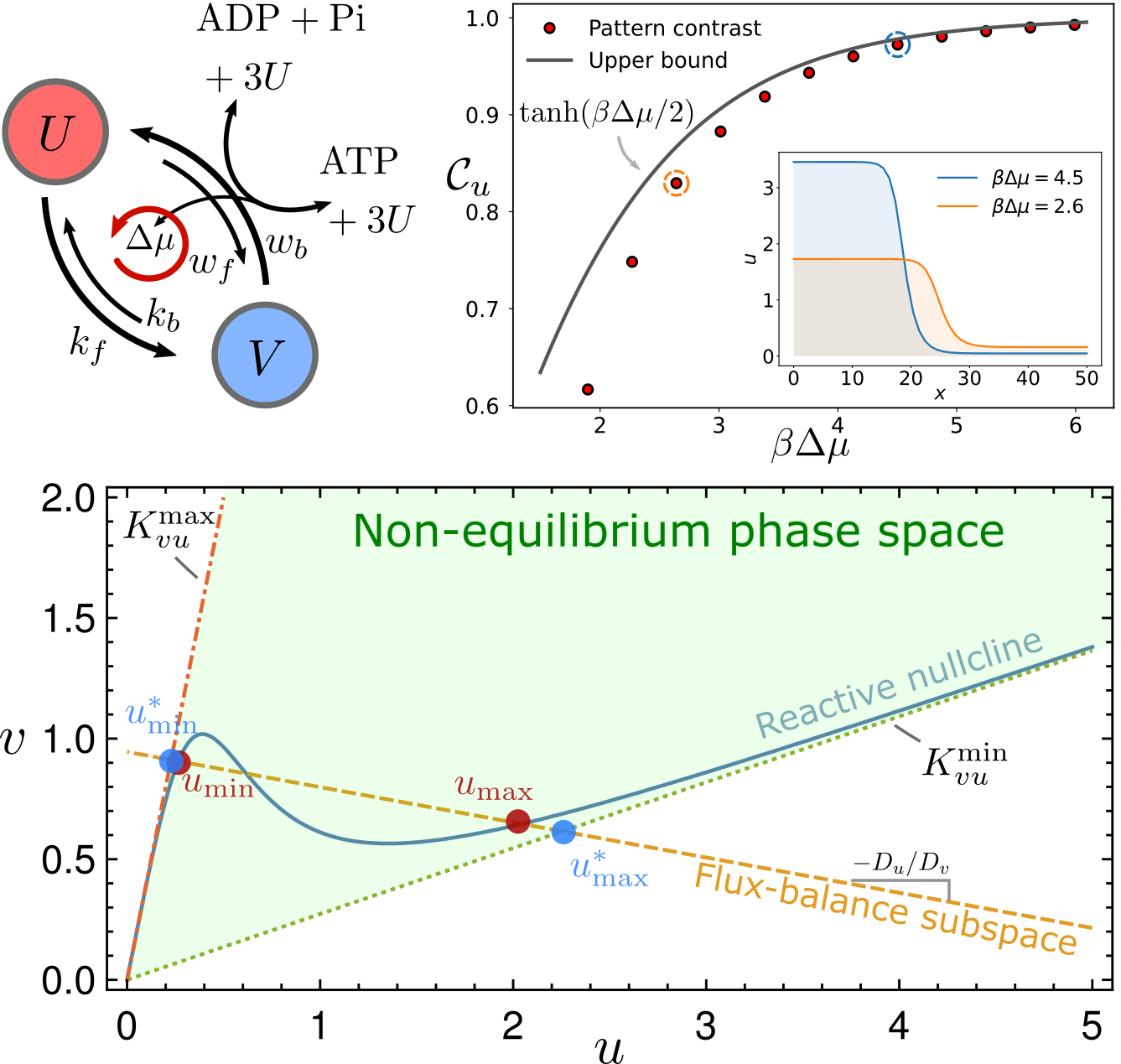
Thermodynamic bounds on symmetry breaking in linear and catalytic biochemical systems
S. Liang, P. De Los Rios & D. M. Busiello
Phys. Rev. Lett. 132, 228402 (2024)
$\color{red}\blacksquare$ Network geometry reveals universal thermodynamic bounds in various of biochemical systems, ranging from the error rate of kinetic proofreading to the contrast of reaction-diffusion pattern.
$\color{red}\square$ Poster | Video
2023
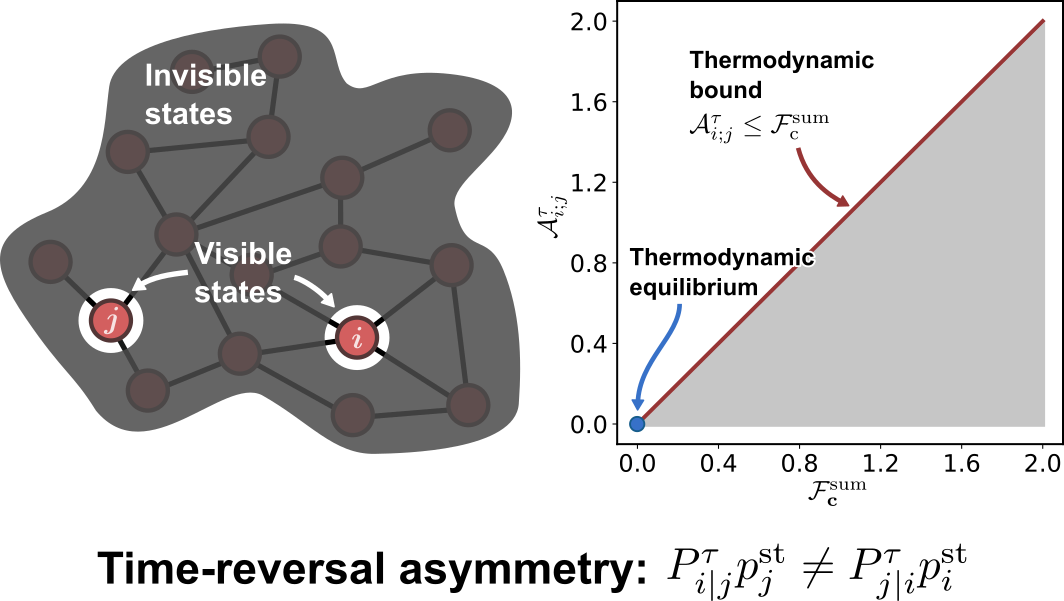
Thermodynamic Bounds on Time-Reversal Asymmetry
S. Liang & S. Pigolotti
Phys. Rev. E 108, L062101 (Letter) (2023)
$\color{red}\blacksquare$ Temporal-coarse-grained measures of time-reversal asymmetry can be used to infer non-equilibrium driving forces.
2022
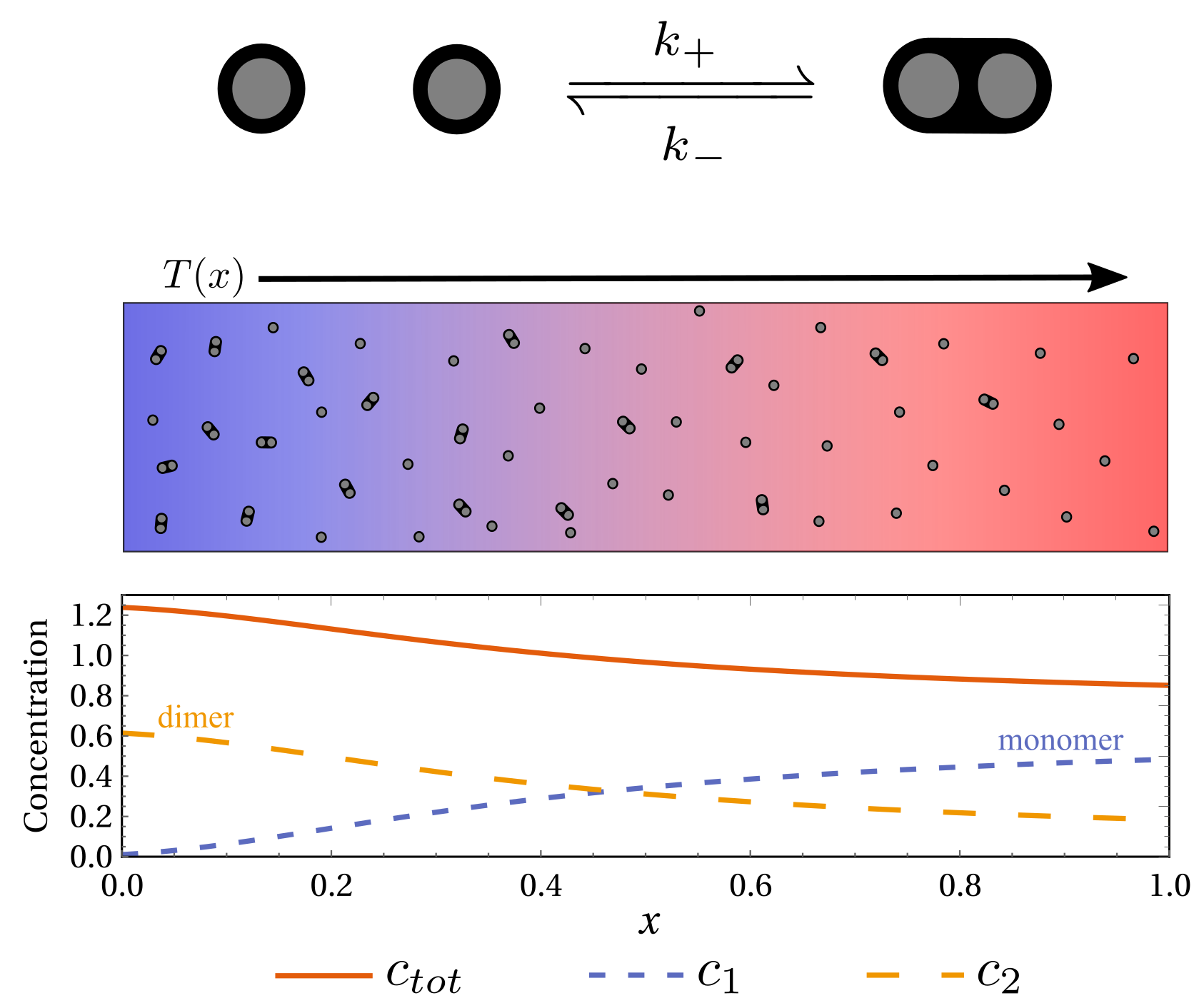
Emergent thermophoretic behavior in chemical reaction systems
S. Liang, D. M. Busiello & P. De Los Rios
New Journal of Physics 24 123006 (2022)
$\color{red}\blacksquare$ Thermophoretic behavior can emerge from reaction-diffusion system.
$\color{red}\square$ Poster | Summary
2021
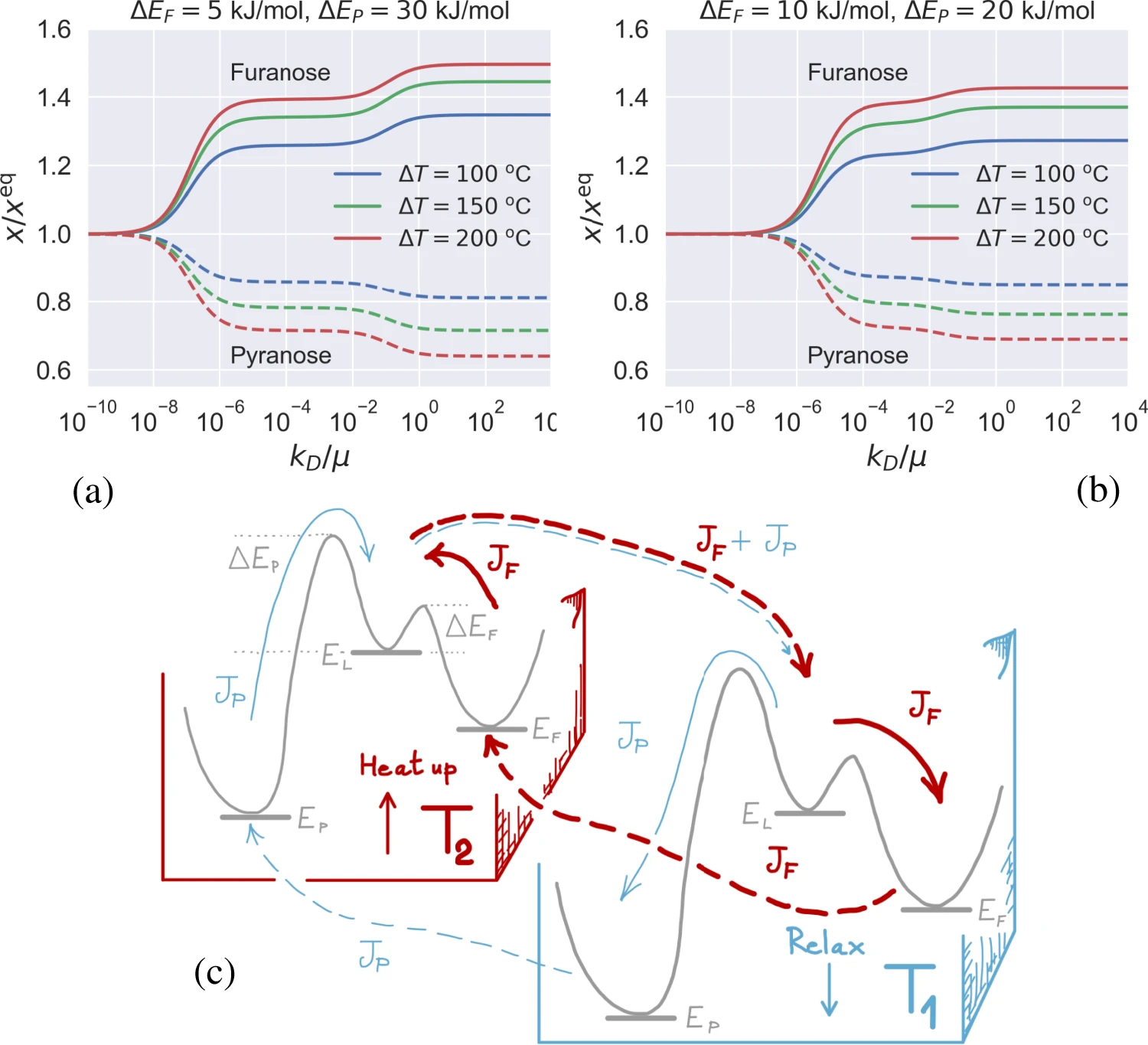
Equilibrium and non-equilibrium furanose selection in the ribose isomerisation network
A. V. Dass, T. Georgelin, F. Westall, F. Foucher, P. De Los Rios, D. M. Busiello, S. Liang & F. Piazza
Nature Communication, 12, 2749 (2021)
$\color{red}\blacksquare$ Temperature gradient can boost the selection of furanose beyond its equilibrium limit.
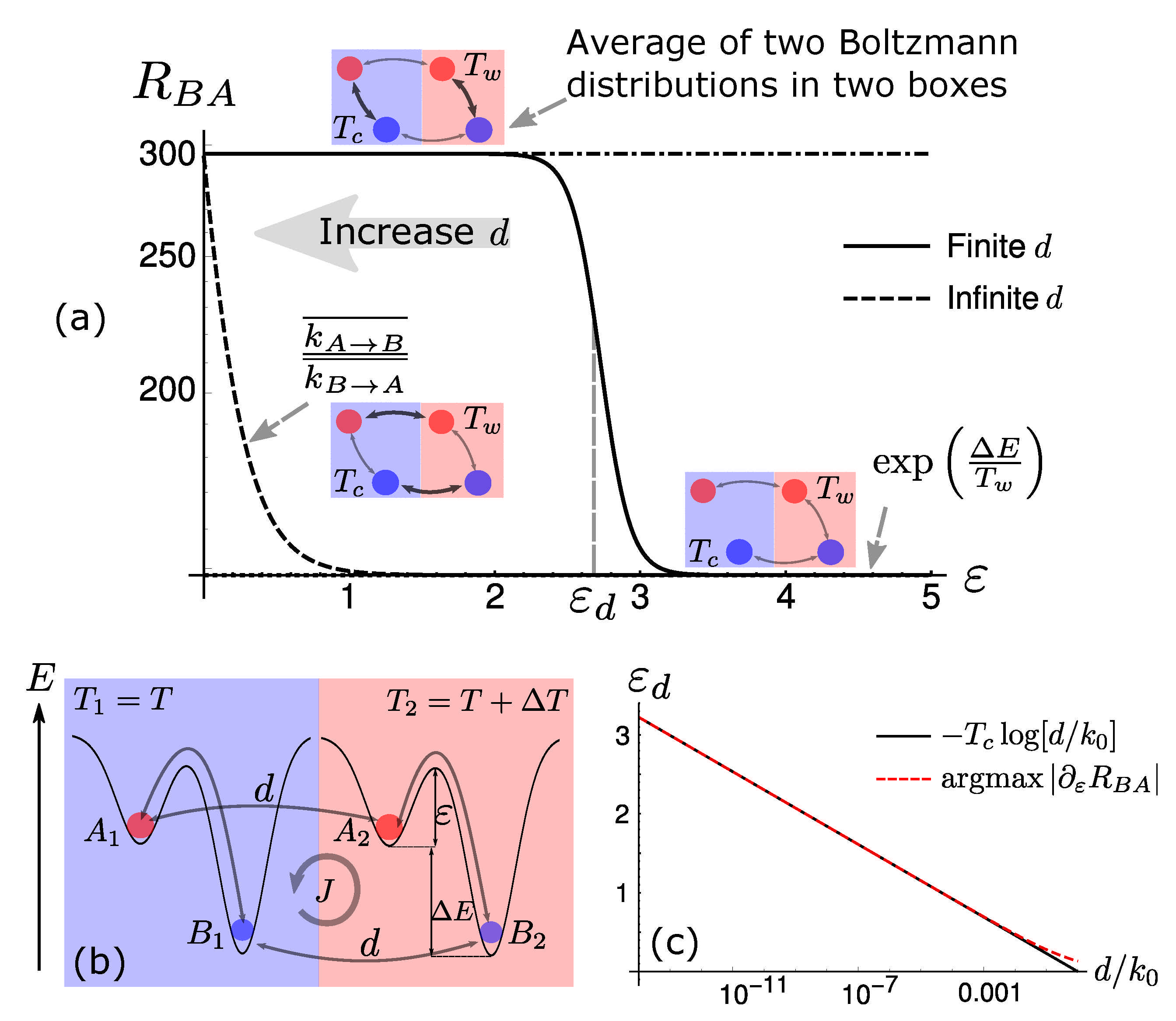
Dissipation-Driven Selection under Finite Diffusion: Hints from Equilibrium and Separation of Time Scales
S. Liang, P. De Los Rios & D. M. Busiello
Entropy 23 1068 (2021)
$\color{red}\blacksquare$ Separation of time scales helps us understand dissipation-driven selection.
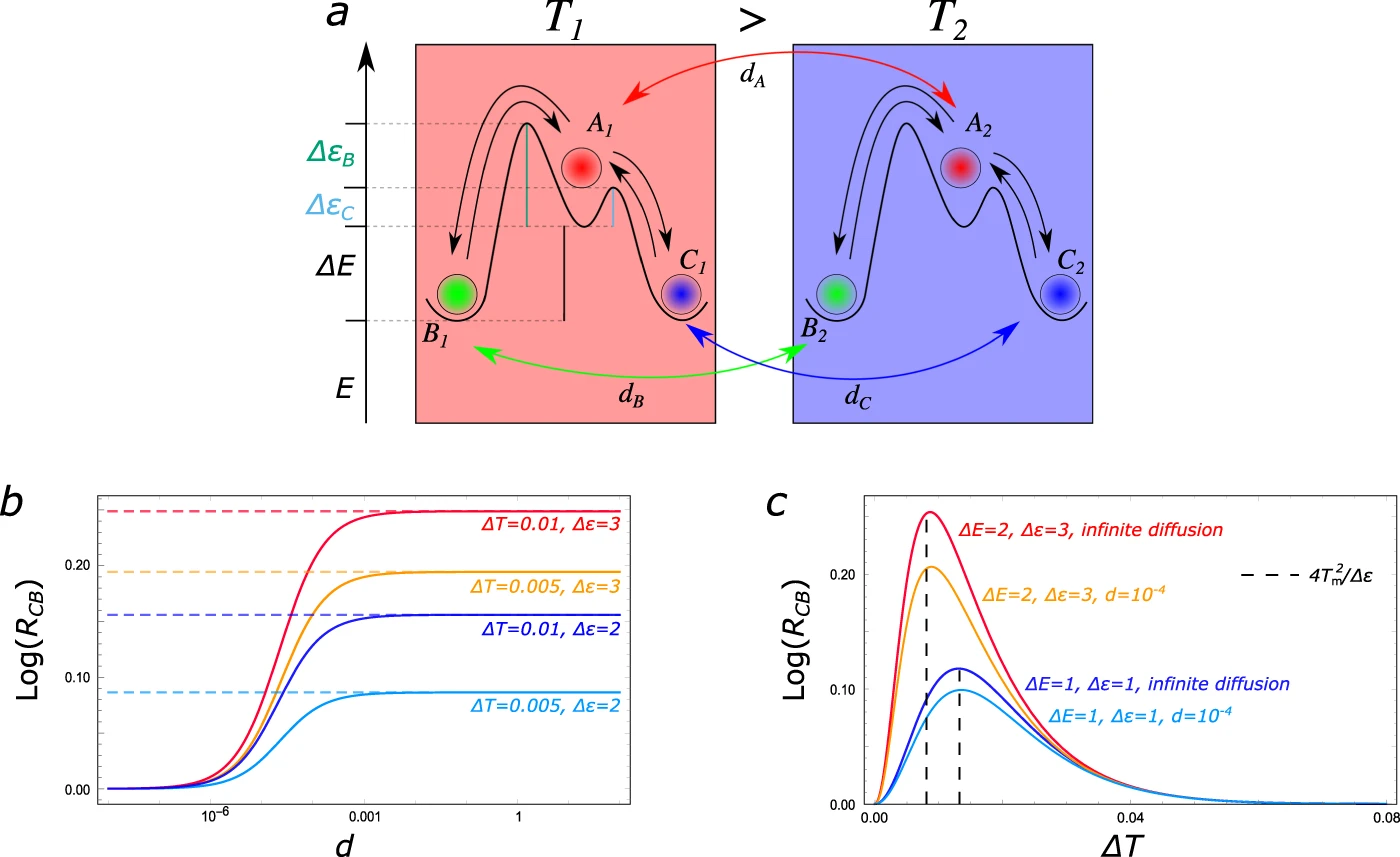
Dissipation-driven selection of states in non-equilibrium chemical networks
D. M. Busiello, S. Liang, F. Piazza & P. De Los Rios
Communication Chemistry 4 16 (2021)
$\color{red}\blacksquare$ Chemical reaction network with kinetic asymmetry can harvest thermal energy to break symmetry in chemical space.
