About me
Hello! I’m Shiling Liang1, an ELBE Postdoctoral Fellow at CSBD and Max Planck Institutes (PKS/CBG) in Dresden, Germany. I received my Ph.D. in Physics from EPFL in 2024, following my M.Sc. in 2020. Prior to that, I completed a joint B.Sc. program in Physics between Beijing Normal University and the University of Manchester in 2018.
My main research interest lies at the intersection of non-equilibrium thermodynamics and living systems. My work revolves around exploring some fundamental questions, such as:
- What general constraints govern systems far from equilibrium?
- How do these constraints shape and limit the behavior of living systems?
- How can we challenge and refine our conventional intuitions on thermodynamics?
If my research resonates with you and you'd like to discuss it further, please don't hesitate to email me.
News
2025.01.13 Our work on heat engine is online on PRL.
2024.12.27 A new preprint on nonequilibrium response theory is on arXiv, comments are welcome!
2024.12.17 A new review, Stochastic thermodynamics for biological functions, is online.
2024.10.27 Starting November 2024, I will begin my postdoctoral research as an ELBE Fellow at CSBD/MPI-PKS/MPI-CBG in Dresden, Germany.
Selected Publications
$\color{black} \blacktriangleright$ Nonequilibrium Nonlienar Response Theory
When we measure physical systems, we apply perturbations and observe how they respond. Our research establishes new mathematical relationships and fundamental limits for these response behaviors in stochastic systems, particularly for non-equilibrium systems where classical equilibrium relationships (like the fluctuation-dissipation theorem) break down.
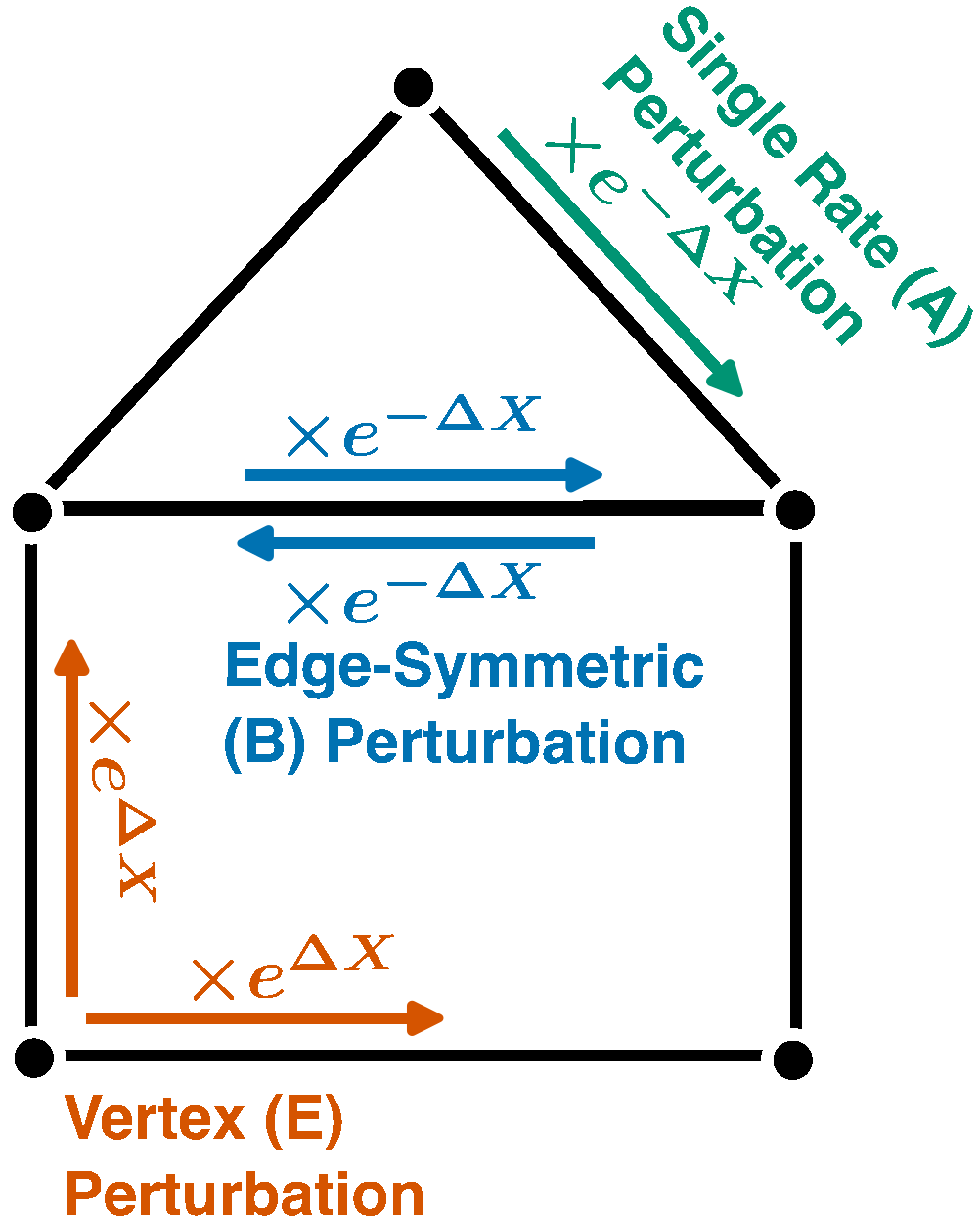
Nonlinear Response Identities and Bounds for Nonequilibrium Steady States
R. Bao$^✉$ & S. Liang$^✉$
arXiv: 2412.19602 (2024)
$\color{red}\blacksquare$ Equalities and bounds for (nonequilirbium) nonlinear resposne of Markov chains under strong perturbation.
$\color{black} \blacktriangleright$ Finite-Time Thermodynamics
Real-world thermodynamic processes operate in finite time, leading to irreversible entropy production. This creates fundamental trade-offs between thermodynamic costs (dissipation) and functional benefits (e.g. power). Our research investigates these trade-offs in finite-time thermodynamics, uncovering their origins and developing principles for optimal design.
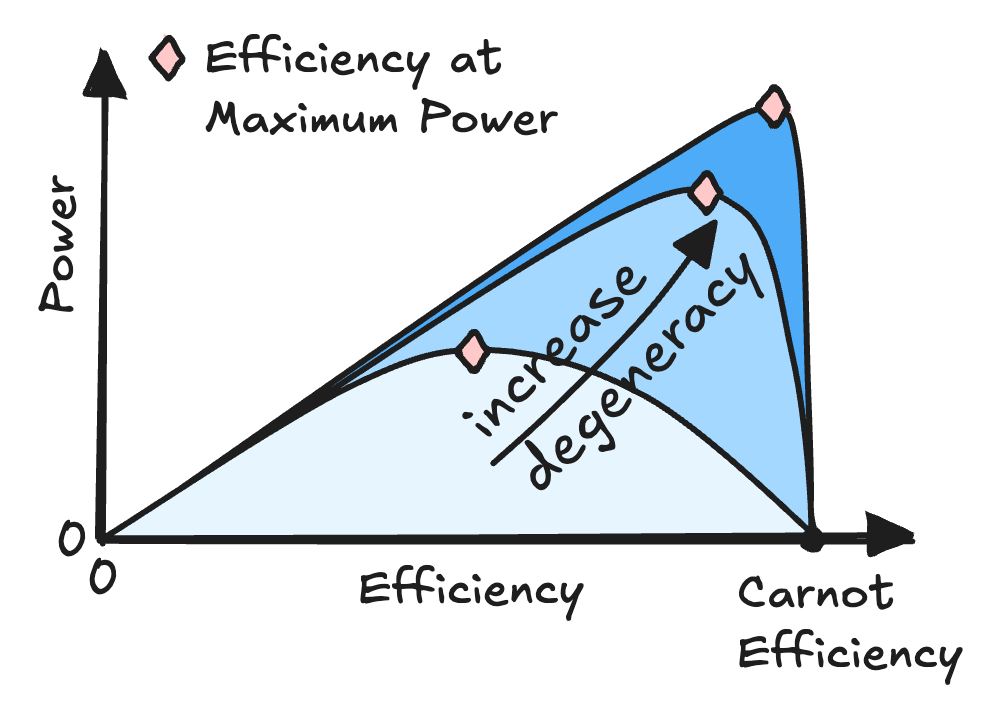
Minimal Model for Carnot Efficiency at Maximum Power
S. Liang$^✉$, Y.-H. Ma$^✉$, D. M. Busiello & P. De Los Rios
Phys. Rev. Lett. 134, 027101 (2025) | arXiv 2312.02323 (2023)
Media Coverge: Phys.org | Interesting Engineering | South China Morning Post
$\color{red}\blacksquare$ A minimal model reveal the attainability of Carnot efficiency at maximum power.
$\color{black} \blacktriangleright$ Thermodynamic Costs of Symmetry Breaking
We develop a general framework for understanding non-equilibrium systems through competing equilibrium states. This approach reveals fundamental thermodynamic constraints on spatial and temporal symmetry breaking in chemical and stochastic processes, providing a unified perspective on how thermodynamic costs bound the emergence of order in living systems.
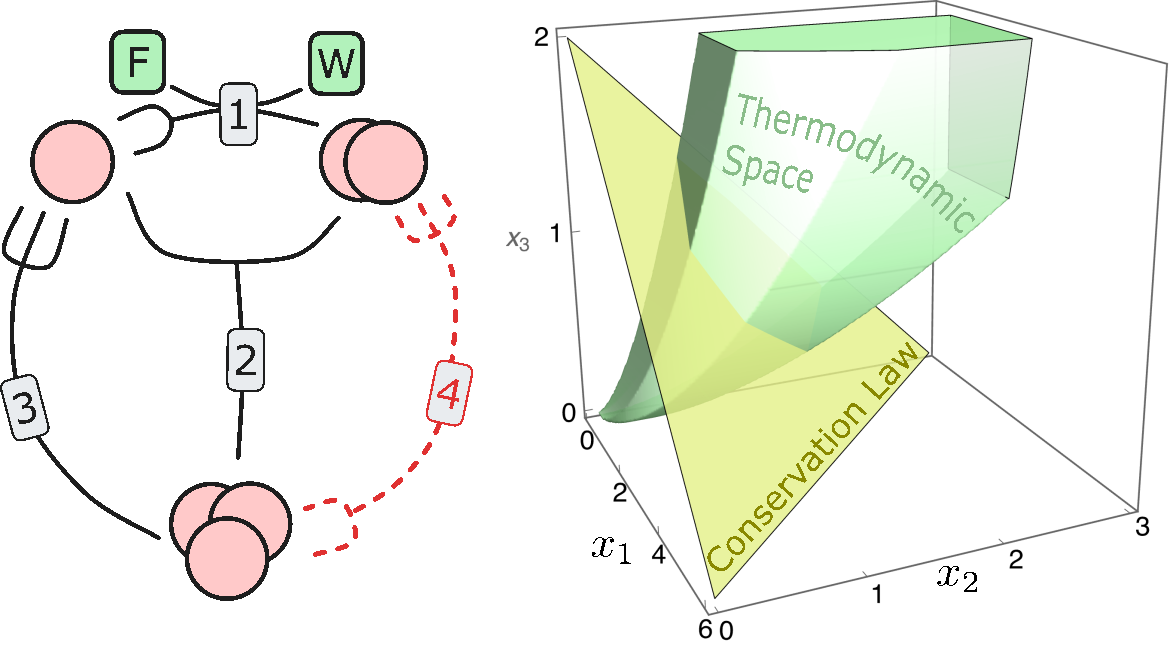
Thermodynamic Space of Chemical Reaction Networks
S. Liang$^✉$, P. De Los Rios & D. M. Busiello$^✉$
arXiv: 2308.14497 (2024)
Video
$\color{red}\blacksquare$ Thermodynamically accessible phase space for (non-equilibrium) chemical reaction networks at stationarity.
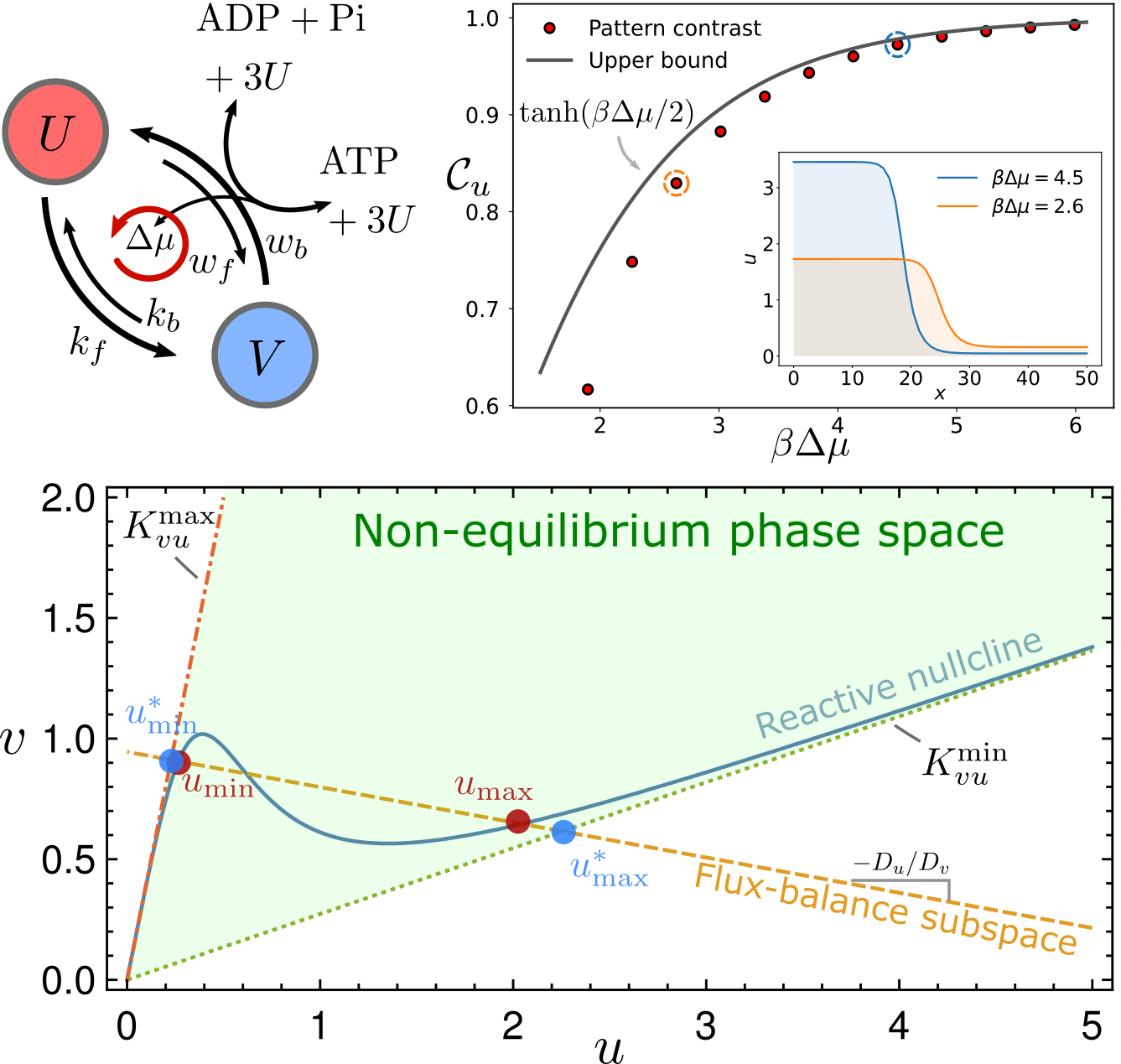
Thermodynamic bounds on symmetry breaking in linear and catalytic biochemical systems
S. Liang$^✉$, P. De Los Rios & D. M. Busiello$^✉$
Phys. Rev. Lett. 132, 228402 (2024)
Poster | Video
$\color{red}\blacksquare$ Network geometry reveals universal thermodynamic bounds in various of biochemical systems, ranging from the error rate of kinetic proofreading to the contrast of reaction-diffusion pattern.
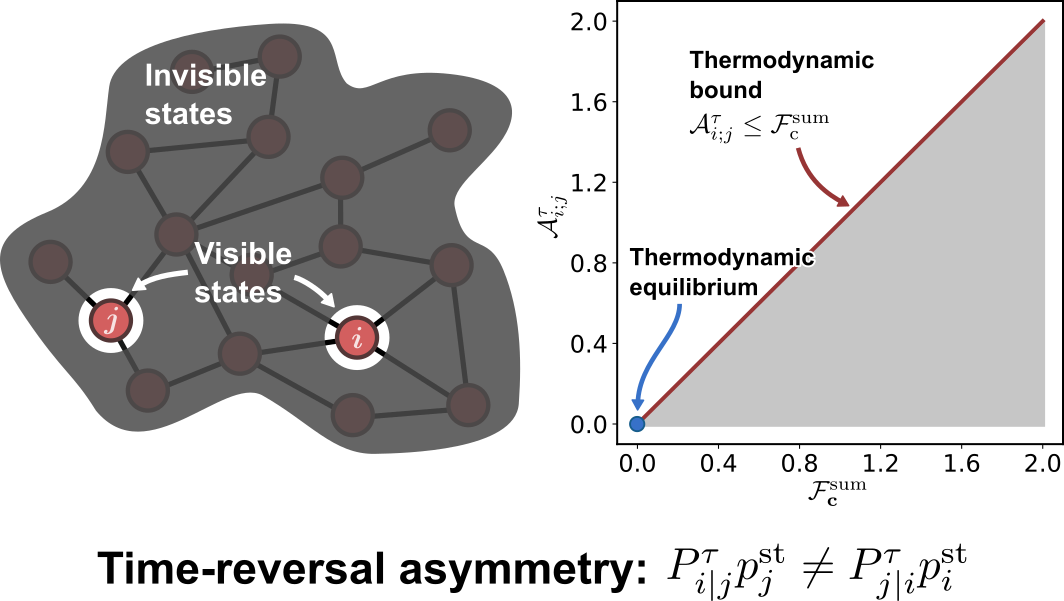
Thermodynamic bounds on time-reversal asymmetry
S. Liang & S. Pigolotti
Phys. Rev. E 108, L062101 (Letter) (2023)
$\color{red}\blacksquare$ Temporal-coarse-grained measure of time-reversal asymmetry can be used to infer non-equilibrium driving forces.
