About me
My name is Shiling Liang1. I am an ELBE Postdoctoral Fellow at CSBD/MPI-PKS/MPI-CBG in Dresden, Germany. I obtained my Ph.D. and M.Sc. in Physics from EPFL, and completed my B.Sc. in Physics through a joint program between Beijing Normal University and the University of Manchester.2.
My research focuses on non-equilibrium thermodynamics and its applications to biochemical systems. During my master’s studies and early PhD work, I investigated how temperature gradients can break symmetries in both chemical and real space, potentially contributing to understanding the origin of life. In my PhD research, I established a general framework for understanding the thermodynamic cost of breaking symmetries in biochemical systems. My interests also include stochastic thermodynamics and thermo-kinetic bounds in stochastic and out-of-equilibrium systems. I approach scientific problems by developing minimal models to understand complex phenomena.
If my research resonates with you and you'd like to discuss it further, please don't hesitate to email me.
News
2024.10.27 Starting November 2024, I will begin my postdoctoral research as an ELBE Fellow at CSBD/MPI-PKS/MPI-CBG in Dresden, Germany.
2024.07.17 The thermodynamics bounds of biochemical systems Phys. Rev. Lett. 132, 228402 got upgraded! Now we can define the thermodynamic space for general chemical reaction networks involving muti-molecular reactions (i.e. hypergraph CRNs). Here is the preprint: arXiv:2407.11498.
2024.05.04 I will attend the Frontiers in Non-equilibrium Physics workshop in Kyoto from 07th to 20th July, 2024.
Selected Publications
$\color{black} \blacktriangleright$ Thermodynamic Costs of Symmetry Breaking

Thermodynamic Space of Chemical Reaction Networks
S. Liang, P. De Los Rios & D. M. Busiello
arXiv: 2308.14497 (2024)
$\color{red}\blacksquare$ Thermodynamically accessible phase space for (non-equilibrium) chemical reaction networks at stationarity.
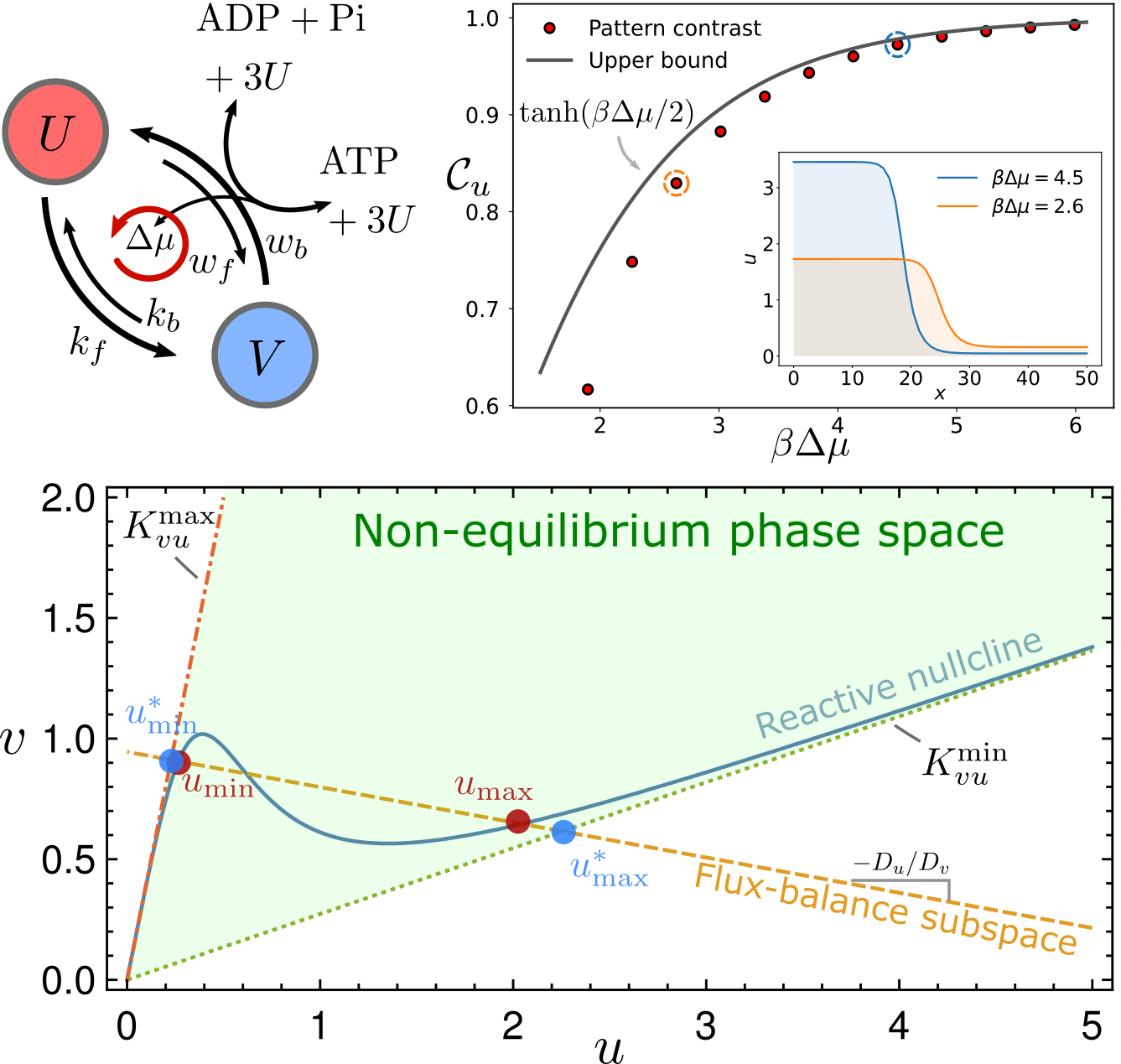
Thermodynamic bounds on symmetry breaking in linear and catalytic biochemical systems
S. Liang, P. De Los Rios & D. M. Busiello
Phys. Rev. Lett. 132, 228402 (2024)
Poster | Video
$\color{red}\blacksquare$ Network geometry reveals universal thermodynamic bounds in various of biochemical systems, ranging from the error rate of kinetic proofreading to the contrast of reaction-diffusion pattern.
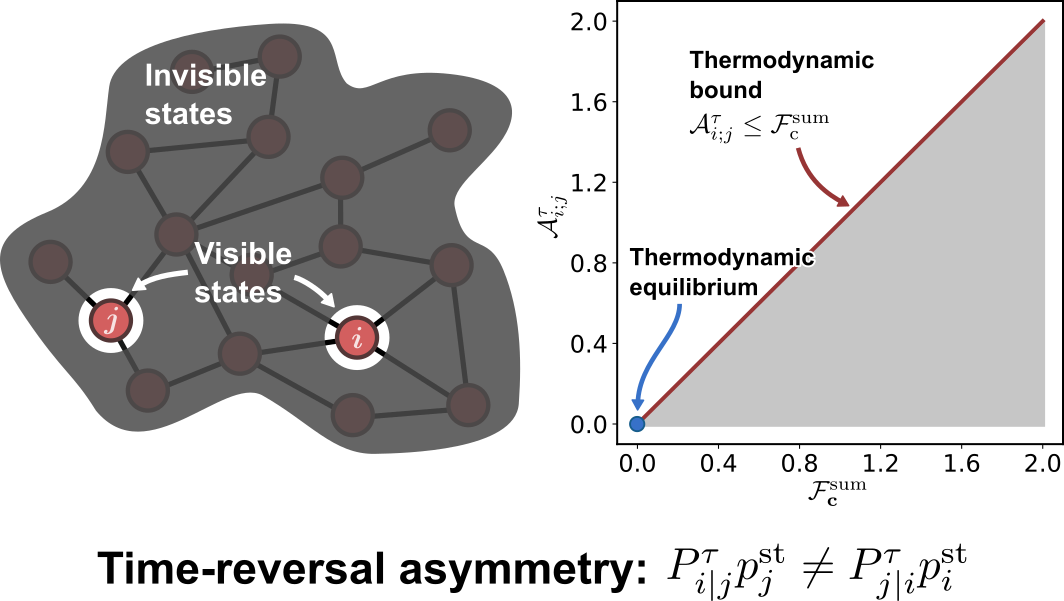
Thermodynamic bounds on time-reversal asymmetry
S. Liang & S. Pigolotti
Phys. Rev. E 108, L062101 (Letter) (2023)
$\color{red}\blacksquare$ Temporal-coarse-grained measure of time-reversal asymmetry can be used to infer non-equilibrium driving forces.
$\color{black} \blacktriangleright$ Finite-Time Thermodynamics
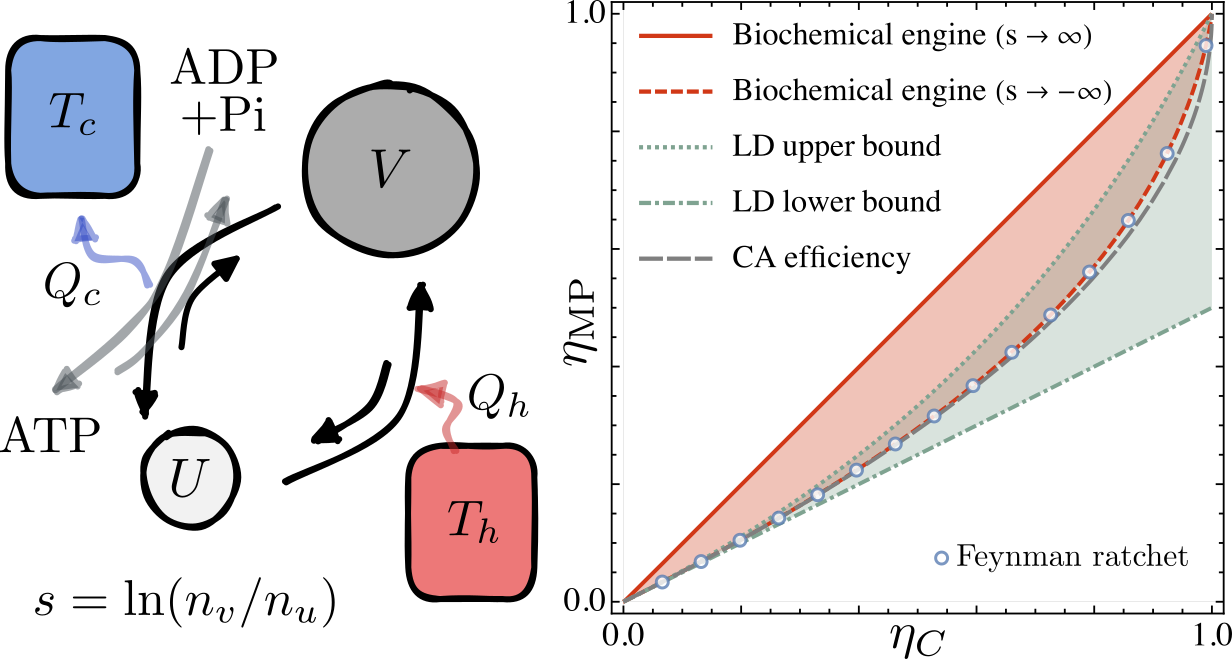
A Minimal Model for Carnot Efficiency at Maximum Power
S. Liang, Y.-H. Ma, D. M. Busiello & P. De Los Rios
Phys. Rev. Lett. (accepted) | arXiv 2312.02323 (2023)
$\color{red}\blacksquare$ A minimal model reveal the attainability of Carnot efficiency at maximum power.
$\color{black} \blacktriangleright$ Chemical Systems in Non-Isothermal Environments
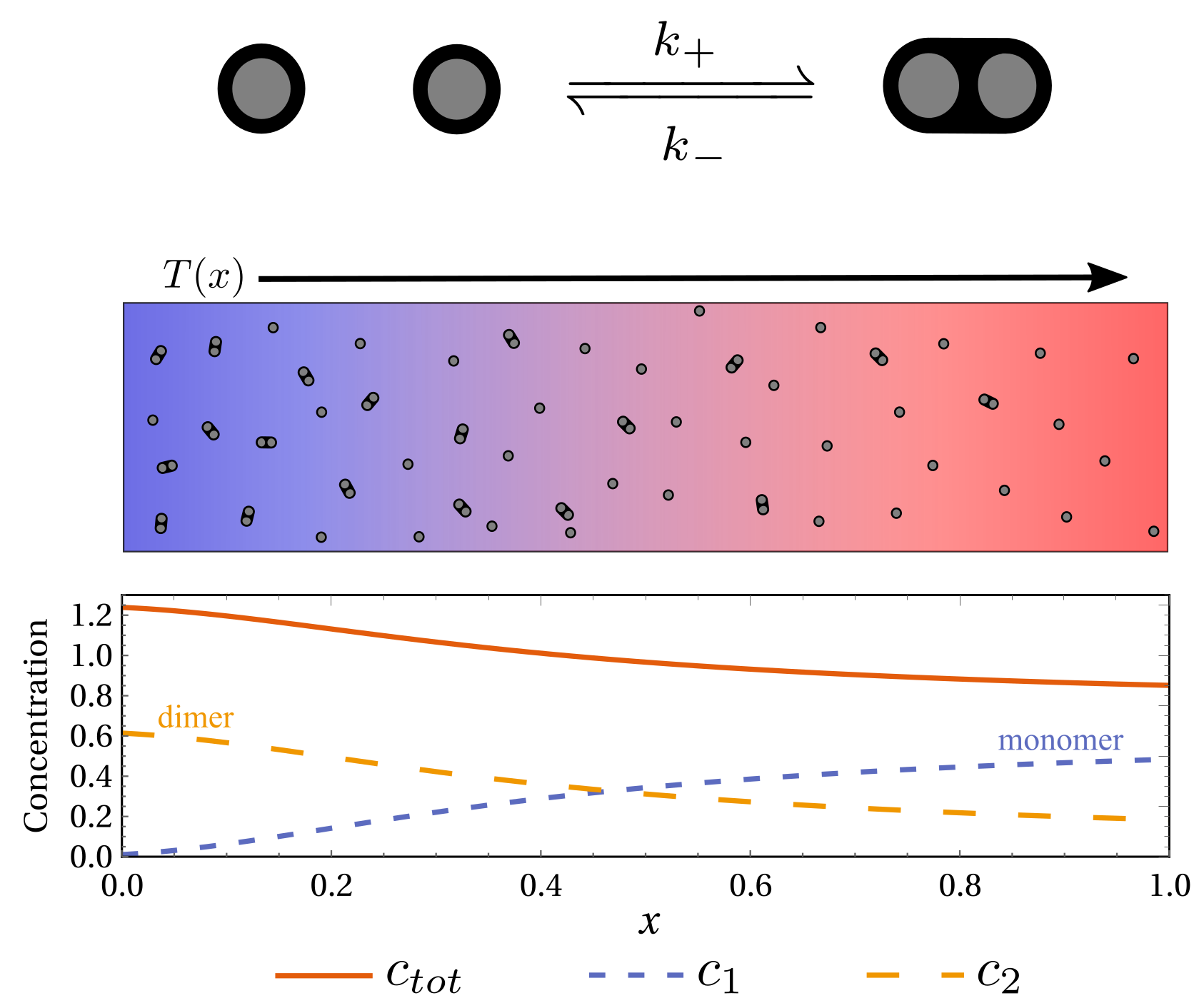
Emergent thermophoretic behavior in chemical reaction systems
S. Liang, D. M. Busiello & P. De Los Rios
New Journal of Physics 24 123006 (2022)
Poster | Summary
$\color{red}\blacksquare$ Thermophoresis can emerge from reaction-diffusion system. Favouring cold or hot regions depends on the correlation between transport properties and energies.
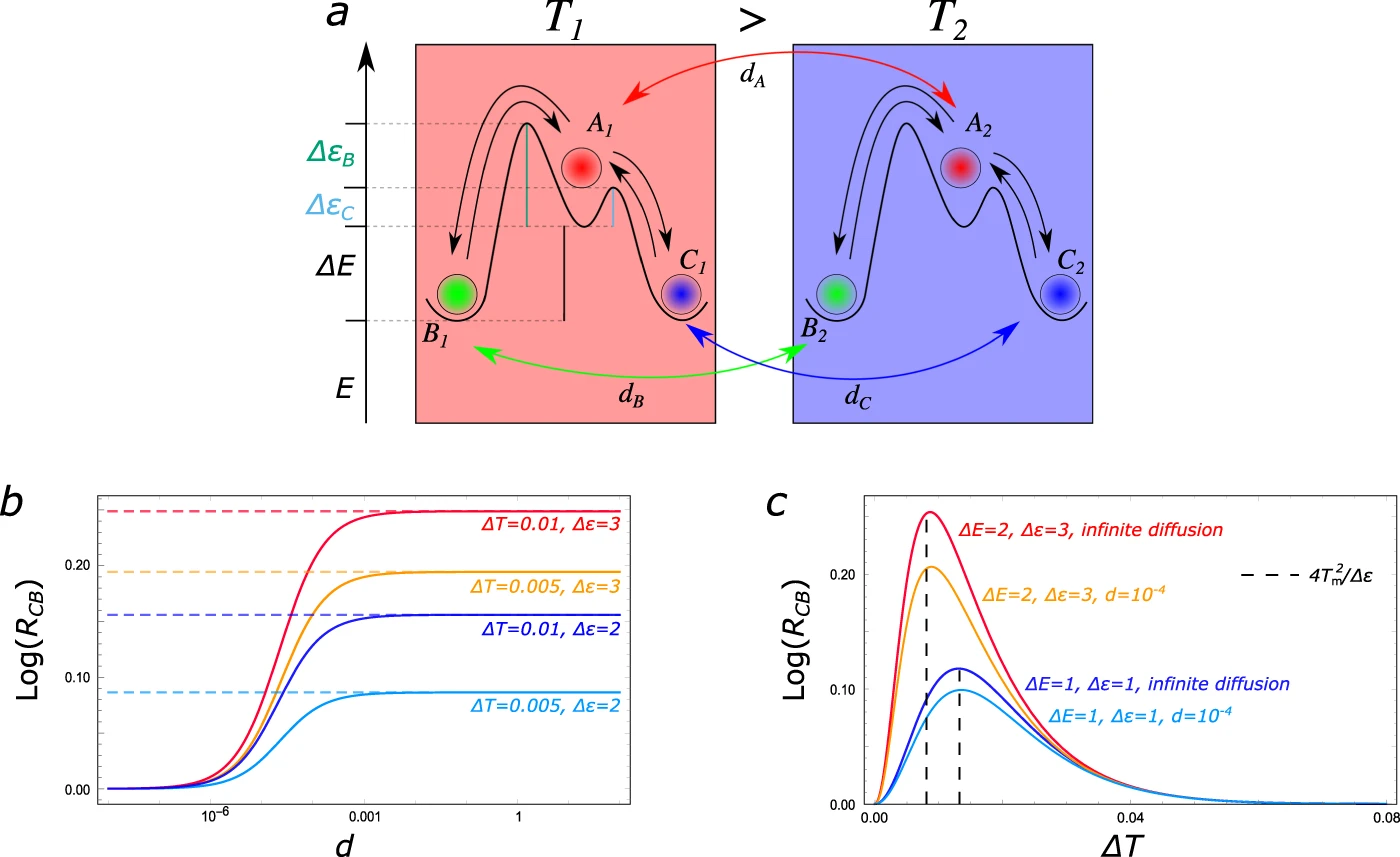
Dissipation-driven selection of states in non-equilibrium chemical networks
D. M. Busiello, S. Liang, F. Piazza & P. De Los Rios
Communication Chemistry 4 16 (2021)
$\color{red}\blacksquare$ Chemical reaction network with kinetic asymmetry can harvest thermal energy to break symmetry in chemical space.
